
Broadening Global Expansion: CreativeBio Draws Attention at ADLM 2024
Colosafe® | Creative Biosciences | 2024.08.02
From July 30 to August 1, 2024, one of the exceptional annual events in the field of global clinical lab testing, the 76th Association for Diagnostics & Laboratory Medicine (ADLM) Annual Clinical Lab Expo, was held in Chicago, USA. Creative Biosciences exhibited in the event, showcasing innovative medical products in DNA testing for cancer and point of care testing.
During the expo, CreativeBio's booth attracted professionals and potential partners from the global medical industry. Company representatives enthusiastically introduced a range of innovative products to attendees from around the world, including DNA test for early detection of cancer, its related automated detection systems, and HemoPOC® coagulation analyzer launched by CreativeBio’s wholly-owned subsidiary called Helixgen.

The CreativeBio team engaged in in-depth exchanges with experts from the international lab medicine, sharing the latest scientific research results and technological advancements, and discussing future trends and challenges in the industry. At this ADLM event, CreativeBio gained over 50 new clients from around the world. These comprehensive exchanges and sharing not only strengthened relationships with existing partners but also expanded new markets in Panama, Argentina, and Uruguay, laying a solid foundation for future global collaborations.


Notably, CreativeBio was the only biotech company at the exhibition offering early detection test for colorectal cancer. Throughout the exhibition, visitors from around the world expressed interest in Colosafe®, a stool DNA test for early detection of colorectal cancer. This technology helps doctors identify early cancerous lesions in patients by interpreting genetic abnormalities in tumor cells shed in the stool, with the potential to prevent colorectal cancer at an early stage, thereby achieving the prevention and eradication of colorectal cancer.
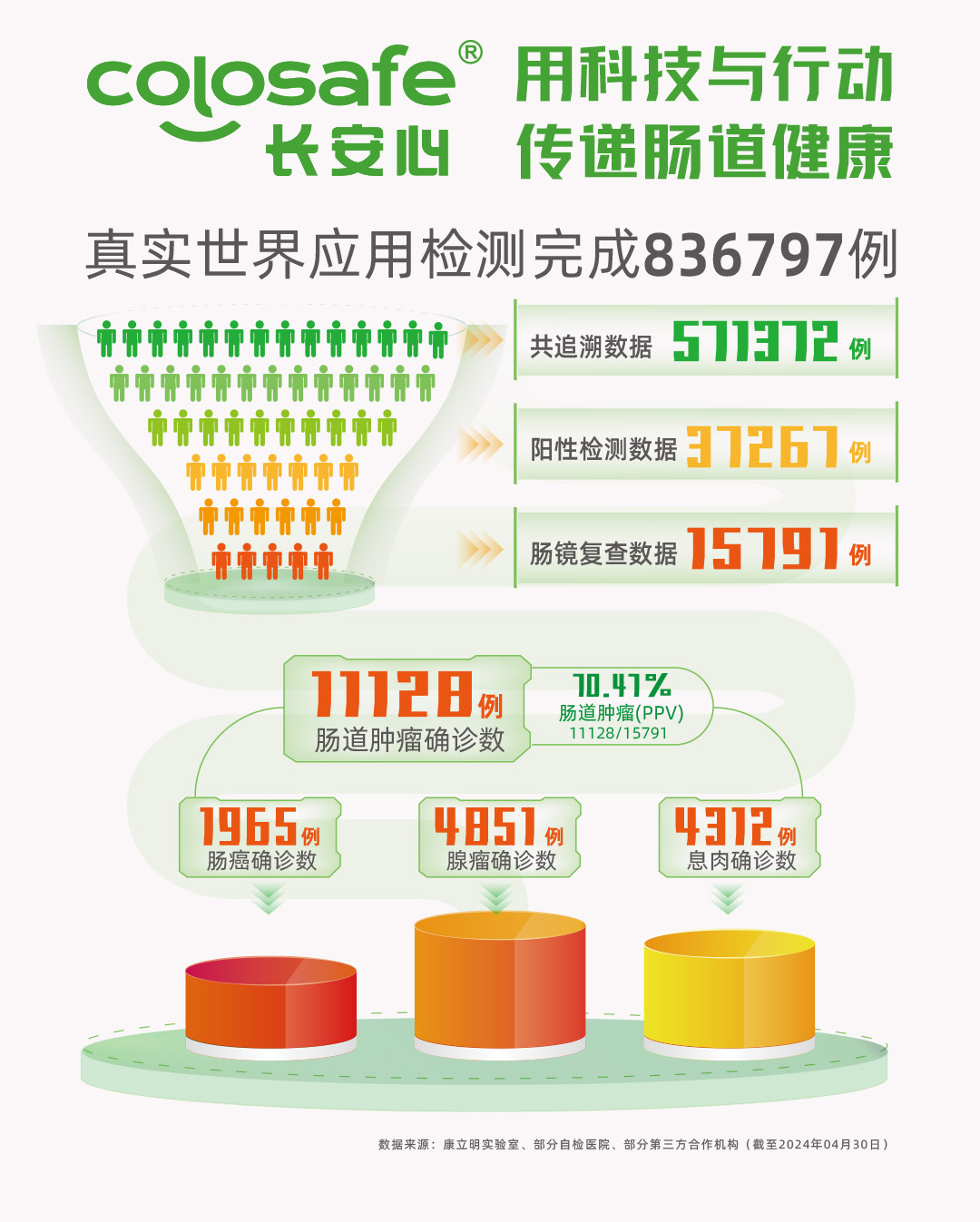
As of April 30, 2024, Colosafe® has completed 836,797 tests, with trackable data of 571,372 cases. Among these trackable cases, 37,267 tests returned positive results (a positivity rate of 6.52%), and colonoscopy follow-up was recommended for all. Of those who tested positive, 15,791 underwent colonoscopy (with a compliance rate of 42.37%), revealing 11,128 individuals requiring treatment. This group included 1,965 cases of colorectal cancer, 4,851 of adenoma, and 4,312 of polyp. The positive predictive value (PPV) for colorectal cancer was 12.44%, and the PPV for colorectal neoplasia was 70.47%, demonstrating excellent diagnostic performance.
In addition to Colosafe®, other innovative products were Automatic Sample Pre-processing Instrument; HemoPOC®, a coagulation testing system; and HelixPOC®, a fully automated nucleic acid detection analyzer, also garnered significant attention from many attendees. HelixPOC®, in particular, attracted a wide crowd of customers from fields such as forensic diagnostics, veterinary medicine, biosafety, and clinical medicine.
The fully automated sample pre-processing instrument is a fecal sample pre-processor that utilizes motor and microcomputer control technology. This allows the rapid and accurate handling of samples, enhancing detection efficiency and of result reliability.

The HemoPOC® coagulation testing system provides convenience for clinical diagnostics by enabling rapid INR/PT testing and warfarin dosage adjustments for patients. This new model offers a more convenient, faster, and accurate approach to anticoagulation management, thereby enhancing the effectiveness of anticoagulation and chronic disease management.

HelixPOC®, fully automated microfluidic fluorescence PCR integrated machine is capable of performing rapid and accurate real-time PCR for qualitative and quantitative analysis, melting curve analysis, and genotyping. It achieves fully enclosed operation and automation from sample preparation to genetic testing results.

Through participation in the ADLM exhibition, CreativeBio successfully demonstrated its cutting-edge technological capabilities in the fields of molecular diagnostics and automation, which evidently enhanced its influence in the global medical technology sector. CreativeBio’s presence was an accomplished brand showcase not to mention a significant representation of Chinese medical technology innovation on the global stage.
CreativeBio will endeavor to uphold its vision of “Human Health, Our Mission” and steadfastly advance onto the international stage, leveraging the power of technology to make greater contributions to safeguarding human health. This exhibition endeavor has injected new momentum into its globalization strategy and will further reinforce the influence and competitiveness of Chinese medical technology worldwide.

